Our Science
MAXIMIZING THE POWER OF MELANOCORTIN AGONISTS
The melanocortin system is mediated by a family of five related receptors that are essential to the body’s control of inflammation, immune response, metabolism, steroid hormone production, and sexual function. This expansive range of effects can be targeted by Palatin’s receptor-selective drugs.
View our science video
Harnessing the Melanocortin System in the Body
Brain
Melanocortin receptors found in the hypothalamus regulate weight maintenance and sexual function, allowing therapeutic interventions that can help people take back control.EYES
Many tissues and immune cells located in the eye express melanocortin receptors, empowering the opportunity to directly activate natural pathways to resolve disease-associated inflammation.Colon
Locally engaging melanocortin receptors found in the intestinal lining and immune cells provides a novel approach to reducing bowel inflammation.Kidney
Kidney cells important to its function express the melanocortin receptor 1 (MCR1). Activating these receptors may improve kidney function and health in a variety of kidney diseases.

We research, design, and develop novel highly selective peptides and small molecule agonists targeted for specific melanocortin receptors.
Our current focus is on the design and development of receptor selective melanocortin agonists for inflammation and autoimmune conditions, with a focus on ocular diseases. Our therapeutics work by activating endogenous melanocortin pathways to resolve damaging inflammation and allow affected tissues time to heal. Research has shown melanocortin agonists can prevent and reverse inflammation in disease models, including in the eyes and intestine—two of our targeted areas.
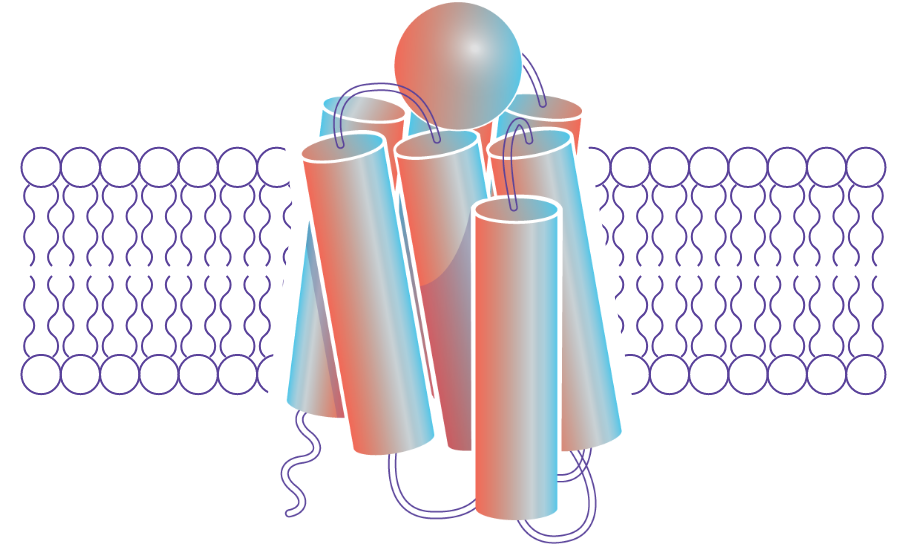

ADVANCING THE TREATMENT OF OCULAR DISEASES
Currently available ocular drugs are hindered by adverse side effects, including poor efficacy, high discontinuation rates, and slow onset of action. Together, these factors create a suboptimal experience, leading to frustration and a need for effective interventions.
Melanocortin receptors are present on the surface of many different cells in the eye, as well as in immune cells residing in the ocular tissue. These melanocortin receptors are a highly tractable target for melanocortin agonists to provide relief and healing for those living with ocular diseases.
LEADING THE WAY IN THE TREATMENT OF AUTOIMMUNE DISEASES
Current treatments for inflammation and autoimmune diseases, such as steroids, immune system modulators, and biologics, broadly suppress immune response. These approaches can impair the body’s immune capabilities on a systemic level, causing adverse side effects and safety concerns.
Melanocortin agonists can potentially resolve inflammation and avoid the adverse side effects and safety concerns of immunosuppressants.
The intestinal lining and resident immune cells express MCR1, and preclinical disease models demonstrate that these receptors can play an important role in controlling colonic inflammation present in ulcerative colitis (UC). Local administration via oral dosing allows our melanocortin agonist to interact with the receptors found on the apical surface of the colon and avoid systemic absorption, and therefore, any associated concerns.
EXTENSIVE CLINICAL PIPELINE
Find out how we continue to leverage our melanocortin agonist expertise to address the unmet needs of patients living with inflammatory and autoimmune diseases.
VYLEESI® (bremelanotide injection)
Visit Vyleesi.com or view Full Prescribing Information to learn more.
INDICATION
VYLEESI is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD), as characterized by low sexual desire that causes marked distress or interpersonal difficulty and is NOT due to:
- A co-existing medical or psychiatric condition,
- Problems with the relationship, or
- The effects of a medication or drug substance
Acquired HSDD refers to HSDD that develops in a patient who previously had no problems with sexual desire. Generalized HSDD refers to HSDD that occurs regardless of the type of stimulation, situation or partner.
Limitations of Use
- VYLEESI is not indicated for the treatment of HSDD in postmenopausal women or in men.
- VYLEESI is not indicated to enhance sexual performance.
Important Safety Information
CONTRAINDICATIONS
VYLEESI is contraindicated in patients who have uncontrolled hypertension or known cardiovascular disease.
WARNINGS AND PRECAUTIONS
Transient Increase in Blood Pressure and Decrease in Heart Rate: VYLEESI transiently increases blood pressure and reduces heart rate after each dose. Advise patients that these changes usually resolve within 12 hours. VYLEESI is not recommended in patients at high risk for cardiovascular disease. Consider the patient’s cardiovascular risk before initiating VYLEESI and periodically during treatment and ensure blood pressure is well-controlled. To minimize the risk of more pronounced blood pressure effects, patients should not take more than one VYLEESI dose within 24 hours. Patients should not use more than 8 VYLEESI doses per month.
Focal Hyperpigmentation: Reported by 1% of patients who received up to 8 doses per month, including involvement of the face, gingiva and breasts. Patients are at higher risk of developing focal hyperpigmentation if they have darker skin and with daily dosing. Resolution of the focal hyperpigmentation was not confirmed in all patients after discontinuation of VYLEESI. Consider discontinuing VYLEESI if hyperpigmentation develops.
Nausea: Reported by 40% of patients who received up to 8 monthly doses, requiring anti-emetic therapy in 13% of patients and leading to premature discontinuation for 8% of patients. Nausea improves for most patients with the second dose. Consider discontinuing VYLEESI or initiating anti-emetic therapy for persistent or severe nausea.
ADVERSE REACTIONS
Most common adverse reactions (incidence >4%) are nausea, flushing, injection site reactions, headache, and vomiting.
VYLEESI may slow gastric emptying and impact absorption of concomitantly administered oral medications. VYLEESI may significantly decrease the systemic exposure of orally-administered naltrexone; avoid use with orally administered naltrexone-containing products intended to treat alcohol or opioid addiction.
PREGNANCY
Advise patients to discontinue VYLEESI if pregnancy is suspected. Advise patients to use effective contraception while taking VYLEESI.
A pregnancy exposure registry monitors pregnancy outcomes in women exposed to VYLEESI during pregnancy. Pregnant women exposed to VYLEESI and healthcare providers are encouraged to call the VYLEESI Pregnancy Exposure Registry at 1-800-972-5220
Please see Full Prescribing Information for Vyleesi® (bremelanotide injection).
INDICATION
VYLEESI is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD), as characterized by low sexual desire that causes marked distress or interpersonal difficulty and is NOT due to:
• A co-existing medical or psychiatric condition,
• Problems with the relationship, or
• The effects of a medication or drug substance.
Acquired HSDD refers to HSDD that develops in a patient who previously had no problems with sexual desire. Generalized HSDD refers to HSDD that occurs regardless of the type of stimulation, situation or partner.
Limitations of Use
• VYLEESI is not indicated for the treatment of HSDD in postmenopausal women or in men.
• VYLEESI is not indicated to enhance sexual performance.
Important Safety Information
CONTRAINDICATIONS
VYLEESI is contraindicated in patients who have uncontrolled hypertension or known cardiovascular disease.
WARNINGS AND PRECAUTIONS
Transient Increase in Blood Pressure and Decrease in Heart Rate: VYLEESI transiently increases blood pressure and reduces heart rate after each dose. Advise patients that these changes usually resolve within 12 hours. VYLEESI is not recommended in patients at high risk for cardiovascular disease. Consider the patient’s cardiovascular risk before initiating VYLEESI and periodically during treatment and ensure blood pressure is well-controlled. To minimize the risk of more pronounced blood pressure effects, patients should not take more than one VYLEESI dose within 24 hours. Patients should not use more than 8 VYLEESI doses per month.
Focal Hyperpigmentation: Reported by 1% of patients who received up to 8 doses per month, including involvement of the face, gingiva and breasts. Patients are at higher risk of developing focal hyperpigmentation if they have darker skin and with daily dosing. Resolution of the focal hyperpigmentation was not confirmed in all patients after discontinuation of VYLEESI. Consider discontinuing VYLEESI if hyperpigmentation develops.
Nausea: Reported by 40% of patients who received up to 8 monthly doses, requiring anti-emetic therapy in 13% of patients and leading to premature discontinuation for 8% of patients. Nausea improves for most patients with the second dose. Consider discontinuing VYLEESI or initiating anti-emetic therapy for persistent or severe nausea.
ADVERSE REACTIONS
Most common adverse reactions (incidence >4%) are nausea, flushing, injection site reactions, headache, and vomiting.
VYLEESI may slow gastric emptying and impact absorption of concomitantly administered oral medications. VYLEESI may significantly decrease the systemic exposure of orally-administered naltrexone; avoid use with orally administered naltrexone-containing products intended to treat alcohol or opioid addiction.
PREGNANCY
Advise patients to discontinue VYLEESI if pregnancy is suspected. Advise patients to use effective contraception while taking VYLEESI.
A pregnancy exposure registry monitors pregnancy outcomes in women exposed to VYLEESI during pregnancy. Pregnant women exposed to VYLEESI and healthcare providers are encouraged to call the VYLEESI Pregnancy Exposure Registry at 1-800-972-5220
Please see Full Prescribing Information for Vyleesi® (bremelanotide injection).